Describe the Difference Between Endothermic and Exothermic Reactions
The final products are stable in exothermic reactions. 5 rows In simple terms the endothermic reactions absorb energy from the surrounding that is in the.

Difference Between Endothermic And Exothermic Reactions
So energy will have to be provided in case of an endothermic reaction but the exothermic reaction does not require energy.
. A curve is drawn up from the energy of the reactants and then down to the energy of the products. Describe the differences between endothermic and exothermic reactions 2. Answer in 2-3 sentences.
Describe the differences between endothermic and exothermic reactions. Describe the difference between an exothermic and endothermic process in terms of a chemical compound. For example when a.
Photosynthesis is a popular example of an endothermic chemical reaction. The key difference between endothermic and exothermic reactions is that endothermic reactions absorb energy from the surrounding environment whereas exothermic reactions release energy to the surrounding environment. Endothermic reactions absorb energy and exothermic reactions release energy.
Compare the slope of the line on a potential energy diagram for an endothermic reaction and an exothermic reaction. Identify chemical reactions as. Describe the difference between an endothermic reaction and an exothermic reaction.
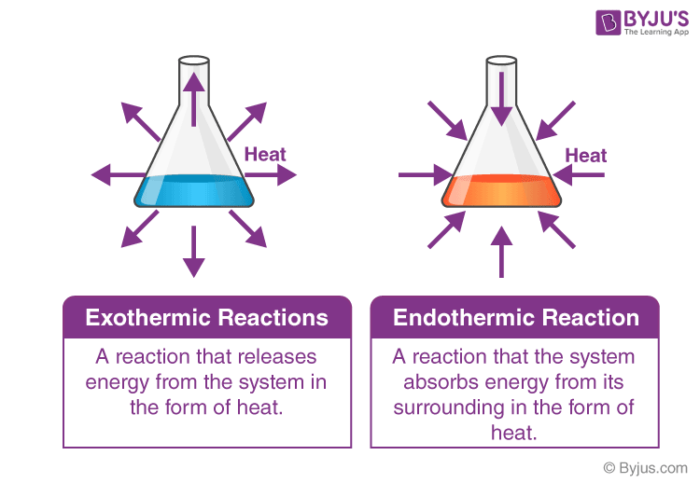
An exothermic reaction releases energy into the environment. A downwards arrow shows that energy is. Describe the difference between an endothermic reaction and an exothermic reaction.
Hỏi x. A Question 5 5 points Listen REQUIRES WORK. Exothermic reactions are reactions or processes that releases energy usually in the form of.
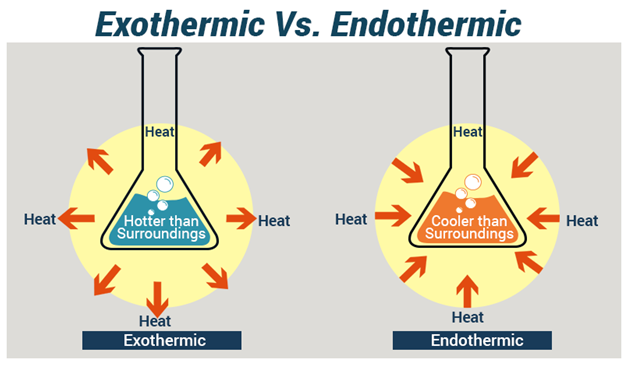
The main difference between endothermic and exothermic reactions is that endothermic reactions absorb energy from the surrounding whereas exothermic reactions release energy to the surrounding. On the other hand an exothermic reaction releases energy into the surrounding of the system. An exothermic reaction has a negative enthalpy difference.
Exothermic and endothermic reactions When a chemical reaction occurs energy is transferred to or from the surroundings. As an example if a process is endothermic with respect to the aqueous solution then DeltaT_sol n 0 the solution gets hotter and it could be due to 1 an exothermic reaction or it could be 2 a liquid vaporizing. Burn coal wood fuel plastic among others.
How does the Law of Conservation of Energy apply to chemical reactions. It can change into other forms such as heat sound light etc. Activity Summary Exothermic and endothermic are common chemical reactions.
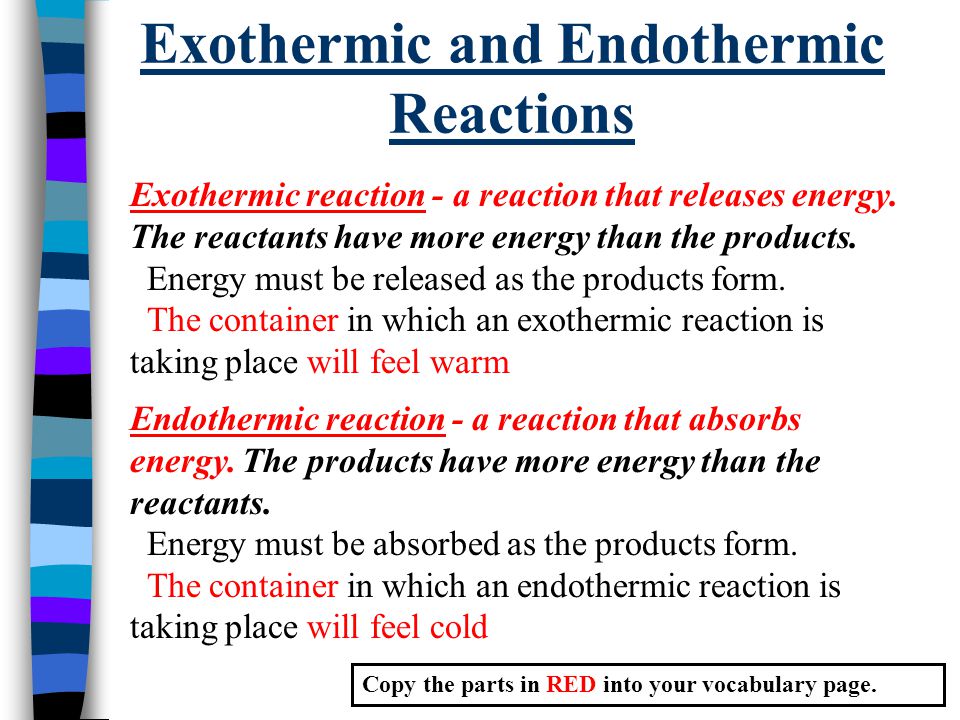
There is usually a temperature change. This is because energy is given out to the surroundings. Anywhere on the left side of the.
Endothermic reactions are those reactions that absorbed energy in the form of heat from the environment whereas exothermic reactions released energy into the surrounding. Chemical reactions are categorized as endothermic and exothermic reactions according to the energy transfer between the system and the surrounding. The energy level decreases in an exothermic reaction.
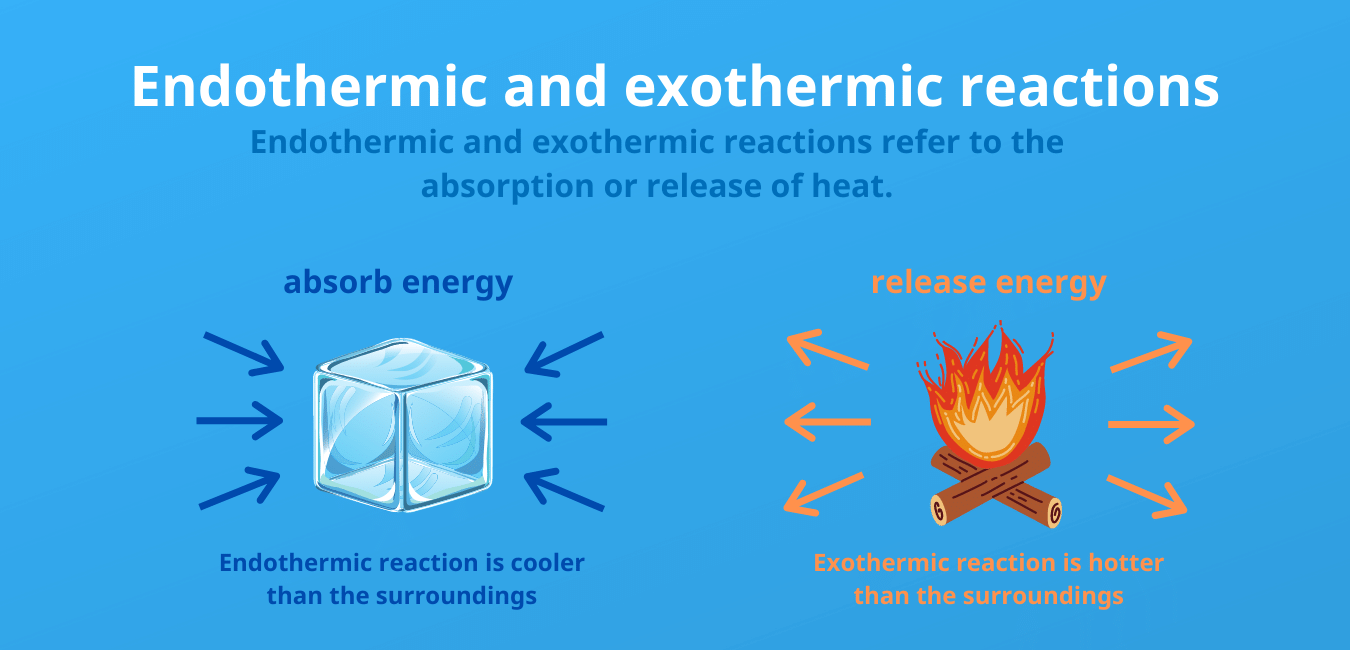
The energy can be absorbed in the form of heat light or sound. An endothermic process absorbs heat and cools the surroundings Based on the above definition lets pick a few examples from our daily lives and categorize them as endothermic or exothermic. Username E-Mail Password Confirm Password Captcha Giải phương trình 1 ẩn.
The correct answer would be They are opposites because endothermic reactions require energy and exothermic reactions release energy. Endothermic and exothermic reactions. NO2 g CO g -- NO g CO2g is second order in NO and zero order in CO at 100 C.
A potential energy diagram is not like an x y ordered pair plot you may be used to in math. 5 rows The major difference between endothermic and exothermic reactions as their names suggest. An exothermic process releases heat causing the temperature of the immediate surroundings to rise.
They are opposites because endothermic reactions require energy and exothermic reactions release energy. Endothermic reactions are those in which energy is given consumed. In this lab students will measure the changes in temperature during reactions and determine what type of reaction has occurred.
The endothermic reactions are when the system takes up the energy in the form of light or heat. These scenarios are typically considered with the following conventions. Differences between exothermic reaction and endothermic reaction.
Endothermic reactions are which absorbs energy from outside the system while exothermic reactions give off or releases energy outside the system. Energy is the capacity to do work. Exothermic reaction in solution.
An experiment shows that the reaction of nitrogen dioxide with carbon monoxide. Describe the difference between an exothermic and endothermic process in terms of a chemical compound or reaction. In a system energy can do work.
The endothermic reactions absorb energy from the surrounding that is in the form of heat. X 2 - 2x 1 -x. Grade Levels 9-12 Learning Objectives 1.
During this process. Answer Expert Verifiedquestion mark.

7 Difference Between Exothermic And Endothermic Reaction With Examples Viva Differences
What Are Exothermic And Endothermic Reactions With Examples Quora

Endothermic And Exothermic Reactions Lab Iteachly Com

Endothermic Vs Exothermic Reactions Chemtalk

Which Of The Reactions Is An Endothermic Reaction Mcq Science

Difference Between Endothermic And Exothermic Reactions
Key Question What Is The Difference Between Exothermic And Endothermic Reactions Warm Up Name 2 Ways You Could Speed Up A Chemical Reaction Ppt Video Online Download
Determination Of The Enthalpy Instruments And Method
Endothermic And Exothermic Reactions Lessons Blendspace
Difference Between Endothermic And Exothermic Reactions Definition Properties Examples

Exothermic And Endothermic Reactions Definition Examples And Differences
Difference Between Endothermic And Exothermic Reactions Definition Properties Examples

Difference Between Endothermic And Exothermic Reactions Compare The Difference Between Similar Terms
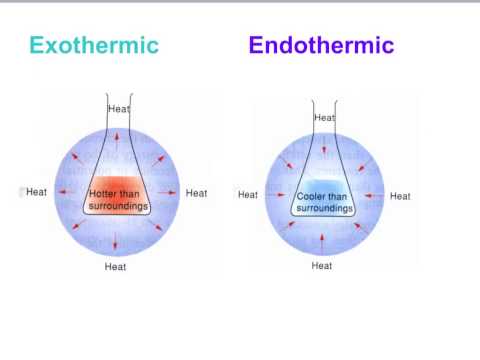
Bond Energy Exothermic And Endothermic Reactions Science Online

Difference Between Endothermic And Exothermic Reaction Brainly In
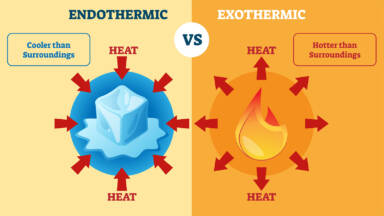
What Does One Mean By Exothermic And Endothermic Reactions Give Examples Cbse Class Notes Online Classnotes123

Differentiate Between Exothermic And Endothermic Reaction

Endothermic Vs Exothermic Reactions Artykul Khan Academy

Key Question What Is The Difference Between Exothermic And Endothermic Reactions Warm Up Name 2 Ways You Could Speed Up A Chemical Reaction Ppt Video Online Download